





Oinkment® spray-on flexi-film bandage kills antibiotic-resistant Staphylococcus to support effective wound care and infection control
Oinkment® spray bandage fills two vital roles in wound care. The sprayable liquid film dries to form a crucial flexible shield over wounds. This initial aspect of wound care is especially helpful for sealing cuts, abrasions, and incisions to prevent further contamination. The film’s non-antibiotic antimicrobial components, embedded in the bandage, fulfill the second key requirement. Killing preexisting contaminating infectious agents is imperative. Unseen and unwelcome, established bacterial contaminants impede wound healing at the direct injury site and often spread systemically to create larger health problems. This study tested the antimicrobial power of Oinkment when applied to three Staphylococcus organisms. The report definitively established that the disinfecting action embedded in Oinkment’s spray film completely sterilized the two harmful antibiotic-resistant S. aureus and a S. hyicus, all pathogens that are ubiquitous among swine farms, within 4 hours of being applied.
DESCRIPTION OF PROBLEM
The problem is more than skin-deep. Efficiently managing swine health requires controlling infections, which generally begin with environmental pathogens first contaminating, then proliferating rapidly in a minor open wound. At the surface, the pathogen’s infectious process blocks natural healing, triggering the wound itself to worsen and enlarge. Below the surface, the infection also spreads systemically.1 Productivity is lost, and the animal’s welfare is undermined.
Arresting a wound’s bacterial contamination at an early stage is a key to effective wound care, fast healing, and preventing systemic complications. Identifying fresh wounds early is vital. Injuries such as umbilical and knee abrasions in young pigs, shoulder and prepuce sores in breeding adults, bite lacerations, as well as cuts from castration and tail docking are infection gateways.2,3,4 The path leads directly to belly ruptures, painful abscesses, joint inflammation, erysipelas, exudative dermatitis (greasy pig disease), and ultimately to profit-robbing carcass downgrades and welfare audits from wholesale meat buyers.5,6,7 Finding and fixing, it is management’s key role.

Yes, antibiotics have been used to treat these infections, but antibiotic success is not assured. Injectable antibiotics are slow to act systemically if they work at all. A report on bacterial samples from 45 swine farms in 5 US swine-producing states documented the commonality of methicillin resistant S. aureus (MRSA), highlighting a key threat to animals and humans.8 The bacteria referred to as “superbugs” were found in swab samples from pigs, from workers on the same farms, and from others in the workers’ households. One of the MRSA used in the present study was selected from among those represented in the swab survey’s findings. This research details the extent to which Oinkment, a spray-on flexi-film bandage containing GRAS OTC microbiocidal components, sterilized two MRSA pathogens, one known to infect both pigs and pig farm workers, and a pig epidermal S. hyicus, the infectious agent responsible for exudative dermatitis or greasy pig disease.
MATERIALS AND METHODS
Researchers tested the antimicrobial effect of Oinkment spray bandage against three pathogenic Staphylococcus bacteria isolates.9
- S. aureus ATCC 6538 MRSA
- S. aureus ATCC BAA-41, MRSA with t002 gene reported in swine and farm workers
- S. hyicus ATCC 11249 isolated from pig epidermis
Procedure: Triplicates of plate count agar were inoculated with 10µl of a 24-hour culture of each of the test bacteria grown in MacFarland suspension. Oinkment was applied to a 47mm diameter area of the bacteria-inoculated plate and the bandage remained in contact for varying durations of 4 hours, 8 hours, and 24 hours. After the contact time elapsed the bandage film was removed, the plate was incubated for 24 hours, then inverted and incubated at 30-35°C for 18-24 hours. The diameter of the original bandaged area was assessed for bacterial growth, along with the area of no bacterial growth extending beyond the edges of the bandaged area. Bandaged plates were compared to non-bandaged control plates inoculated with the same organism and non-inoculated media control plates.10
RESULTS
Inoculated control plates contained copious bacterial overgrowth, too numerous to count, confirming the organism viability and the adequacy of the test conditions. Control plates containing media alone contained no growth, indicating there was no unintended bacteria present in the test system.
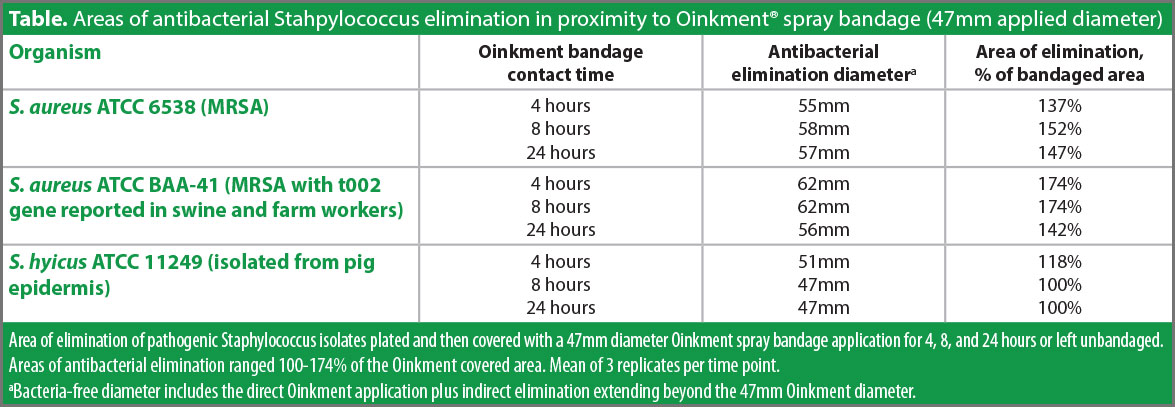
No bacteria remained viable under the area contacted by the 47mm diameter bandage for any of the organisms at any contact duration, exhibiting Oinkment’s antibacterial effect when in direct contact with the underlying pathogenic bacteria (Table).
Beyond the bandage edge for most of the organism and contact time combinations, additional areas of bactericidal effect expanded the bacteria kill zones. These expanded zones demonstrated that the bandage’s antimicrobial impact indirectly reached beyond the area contacted by the bandage material. Specifically, the total area of bacterial elimination of S. aureus ATCC 6538 increased, ranging 137-152% of the area directly under the bandage. The total sterilization area of S. aureus ATCC BAA-41 ranged 142-174% of the treated area. The S. hyicus ATCC 11249 bacteria exhibited less susceptibility to this expansion, ranging 100-118%. The extent of these additional kill zones on the culture plates is a function of each organism’s susceptibility to the antimicrobials migrating through the culture plate media. Such migration and kill zone expansion may not be as extensive in vivo, highlighting the importance of complete wound coverage when spraying the bandage.
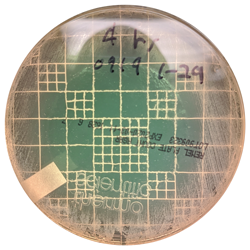
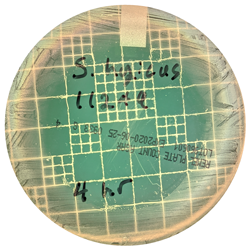
The antibacterial effect was swift as well as pronounced, evidenced by each of the organisms being completely killed at the shortest 4 hour contact time (Figure). This evidence reinforces the importance of quickly spraying the bandage on piglet processing incisions and responding to open cuts and abrasions as soon as they are identified to initiate effective wound care.
CONCLUSIONS AND APPLICATIONS
Accelerating healing through good wound care requires a moist, clean environment, free from contaminants and dead tissue, encouraging fresh cells to infiltrate to the exposed wound. Oinkment’s flexi-film spray supports this need, adhering to skin, conforming to the body shape, supplementing the natural function of a scab (which is frequently abraded off in live production environments), sealing out filth and holding in moisture.
Despite managers tending to these vital physical wound care techniques, a wound that remains contaminated with invisible, often antibiotic-resistant organisms, cannot repair itself optimally. This study definitively established that the non-antibiotic antimicrobial action embedded in Oinkment’s spray film can completely sterilize harmful Staphylococcus organisms common in swine farms within 4 hours of being applied. Safe and non-toxic, the microbiocidal skin protectant, analgesic, and antifungal elements in Oinkment support AMDUCA compliance, being approved as OTC in 21 CFR 310.545. They, along with all other components, are also affirmed as GRAS in the 21 CFR list of direct food substances. The bandage contains no antibiotics and requires no preslaughter withdrawal.
While the wound is healing, the film covering remains in place to protect the bed of fragile fresh cells, helping new tissue stay soft and pliable. The wound remains sealed as the film adheres to the healthy skin surrounding the wound. It is not necessary to remove the patch, but if it is removed gently with moisture or by environmental abrasion, the adhering film will debride the wound, removing dead tissue and dirt to facilitate clean growth. Re-applying the spray patch daily or as needed keeps the area sealed and on-the-mend, contributing positively to animal health, welfare, and farm productivity.
Figure. Photos comparing bacterial growth on control culture plates with no Oinkment® versus plates treated with Oinkment spray bandage applied for 4 hours
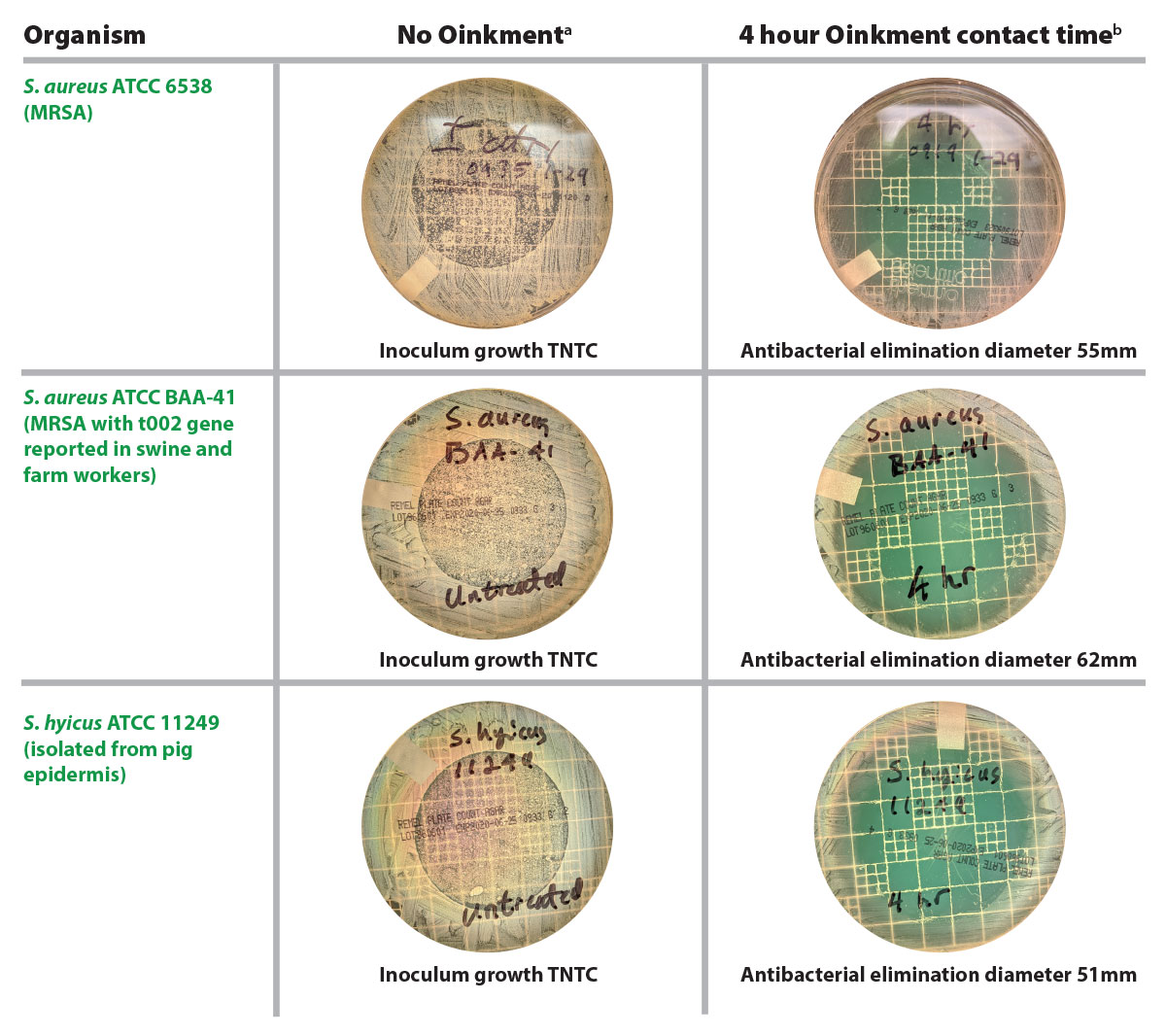
aControl plates demarking the central untreated 47mm diameter target areas and illustrating copious bacterial growth to the plate edge, too numerous to count (TNTC).
bBacteria-free area of plates treated with Oinkment spray bandage. Clear kill zones include the 47mm diameter Oinkment application area plus indirect elimination extending beyond the margins of the Oinkment application. Mean of 3 replicates of each culture plate.
REFERENCES AND NOTES
1. Anonymous. Abscesses. 5m Publishing, Sheffield, England. Accessed online May 2020.
https://thepigsite.com/disease-guide/abscess
2. Meyer, D., M. Hewinker-Trautwein, M. Hartmann, L. Kreienbrock and E. Grosse. 2019. Scoring shoulder ulcers in breeding sows – is a distinction between substantial and insubstantial animal welfare-related lesions possible on clinical examination? Porc Health Manag 5, 3. Accessed online May 2020.
https://porcinehealthmanagement.biomedcentral.com/articles/10.1186/s40813-018-0108-3
3. Anonymous. Preputial ulceration. 5m Publishing, Sheffield, England. Accessed online May 2020.
https://thepigsite.com/disease-guide/preputial-ulcers
4. Anonymous. Vice (tail-biting, flank chewing, ear biting). 5m Publishing, Sheffield, England. Accessed online May 2020.
https://thepigsite.com/disease-guide/vice-abnormal-behaviour-tail-biting-flankchewing-ear-biting
5. Greiner, L. 2012. Understanding umbilical hernias. National Hog Farmer. Accessed online May 2020.
https://www.nationalhogfarmer.com/health/understanding-umbilical-hernias
6. M. Giles. 2019. Small-scale pig keeping: prohibiting zoonotic pathogens. 5m Publishing, Sheffield, England. Accessed online May 2020.
https://thepigsite.com/articles/small-scale-pig-keeping-prohibitingzoonotic-pathogens
7. Edwards, L. 2019. What can pig carcasses tell us about farm welfare? 5m Publishing, Sheffield, England. Accessed online May 2020.
https://thepigsite.com/articles/what-can-pig-carcases-tell-usabout-farm-welfare
8. Smith, T.C., W.A. Gebreyes, M.J. Abley, A.L. Harper, B.M. Forshey, M.J. Male, H.W. Martin, B.Z. Molla, S. Sreevatsan, S. Thakur, M. Thiruvengadam and P.R. Davies. 2013. Methicillin-resistant Staphylococcus aureus in pigs and farm workers on conventional and antibiotic-free swine farms in the USA. PLoS One. Accessed online May 2020.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3646818/
9. Pearce, P.J. 2020. Personal communication. Report of antimicrobial susceptibility assessment performed by Nova Biologicals, Inc. Conroe, Texas, USA.
10. Hudzicki, J. 2009. Kirby-Bauer disk diffusion susceptibility test protocol, American Society for Microbiology. Manual of Microbiology, 9th ed. 2007 FAO JECFA Monograph, vol 4.
Additional Information and Related Articles on Oinkment®
SpecSheet English | SDS | Sell Sheet For use as a topical protective barrier on the skin of swine to shield from abrasion and guard against contamination that may cause infection.
RELATED ARTICLES:
Oinkment Infection Control PDF|Article
The Best Barrier to Protect from Staphylococcus PDF
Mailing Address:
Animal Science Products, Inc
PO Drawer 631408
Nacogdoches, TX 75963 - 1408
Physical Address:
3418 Rayburn Drive
Nacogdoches, Texas 75961
Phone
936.560.0003
